Unique Device Identification - UDI
Tabs - UDI
Unique Device Identification - UDI
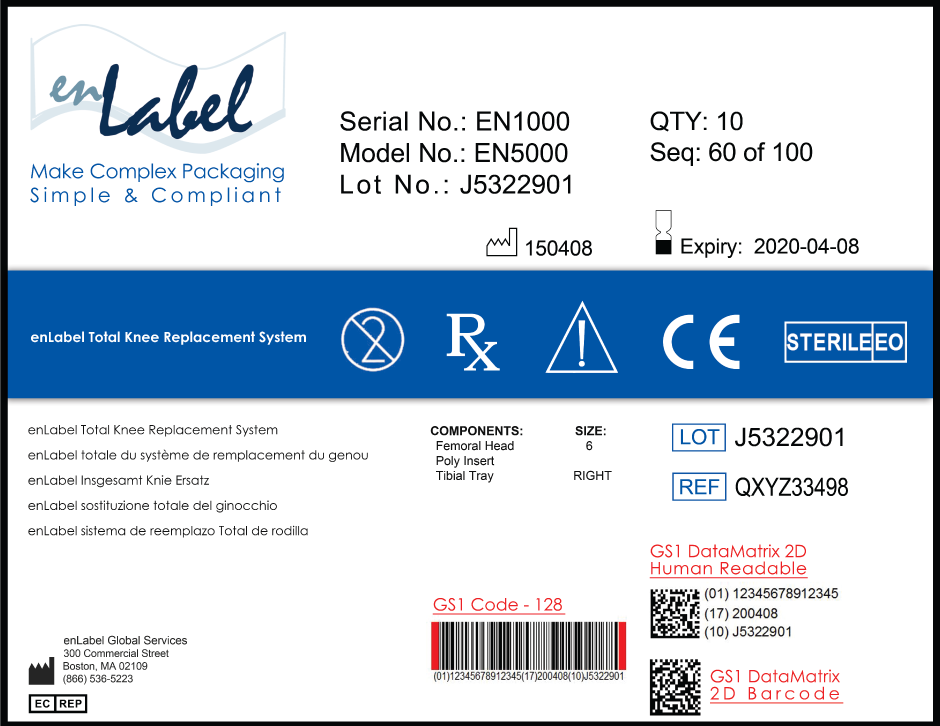
A UDI is a unique numeric or alphanumeric code that consists of two parts:
- a device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device, and
- a production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- the lot or batch number within which a device was manufactured;
- the serial number of a specific device;
- the expiration date of a specific device;
- the date a specific device was manufactured;
- the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
As part of the UDI system, the FDA is also creating the Global Unique Device Identification Database (GUDID) which will include a standard set of basic identifying elements for each device with a UDI. Most of this information will be made available to the public so that users of a medical device can easily look up information about the device. The UDI does not indicate, and the database will not contain, any information about who uses a device, including personal privacy information.
The FDA has issued Global Unique Device Identification Database (GUDID) - Guidance for Industry and FDA Staff (pdf)to give labelers an overview of the GUDID. This guidance is designed to help labelers prepare to submit information to the GUDID by describing key GUDID concepts such as accounts, user roles, the device identifier record life cycle, package configurations, and the GUDID data attributes and descriptions.
Benefits of Unique Device Identification
When fully implemented, the UDI system can:
- Allow more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
- Reduce medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
- Enhance analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries. A more robust post market surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
- Provide a standardized identifier that will allow manufacturers, distributors and healthcare facilities to more effectively manage medical device recalls.
- Provide a foundation for a global, secure distribution chain, helping to address counterfeiting and diversion and prepare for medical emergencies.
- Lead to the development of a medical device identification system that is recognized around the world.
IPM & UDI
Manage UDI standards with minimal impact to your overall manufacturing and quality processes.
Sharply enforced and ever-evolving UDI regulations shed light on existing gaps, workarounds and inefficiencies within labeling processes, across an enterprise.
As a result of uncovering such setbacks, UDI regulations should serve as a catalyst, and business justification, to improve or simplify operational processes.
The enLabel IPM Platform will continuously support UDI standards by allowing you to:
- Dynamically include production specific and product specific details in the identifier (lot + item).
- Use flexible bar coding standards (GS1, EAN, RFID, 2D and 1D) with minimal impact to operations.
- Deploy trace & track capability throughout the entire supply chain, from received goods to distribution to the point of use.
- Prepare for 2016 and beyond with minimal changes to your current manufacturing process.
- Achieve zero defects in your labeling, documentation and packaging processes.
Compliance Dates for UDI Requirements
The table below outlines key compliance dates in the UDI final rule.
| Compliance Date | Requirement |
|---|---|
|
1 year after publication of the final rule (September 24, 2014) |
The labels and packages of Class III medical devices, and devices licensed under the Public Health Service Act (PHS Act) must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. Data for these devices must be submitted to the GUDID database. § 830.300. A 1-year extension of this compliance date may be requested under § 801.55; such a request must be submitted no later than June 23, 2014. Class III stand-alone software must provide its UDI as required by § 801.50(b). |
|
2 years after publication of the final rule (September 24, 2015) |
The labels and packages of implantable, life-supporting, and life-sustaining devices must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. |
|
A device that is a life-supporting, or a life-sustaining device that is required to be labeled with a UDI must a bear UDI as a permanent marking on the device itself if the device is intended to be used more than once and intended to be reprocessed before each use. § 801.45. Stand-alone software that is a life-supporting or life-sustaining device must provide its UDI as required by § 801.50(b). |
|
|
Data for implantable, life-supporting, and life-sustaining devices that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. |
|
|
3 years after publication of the final rule (September 24, 2016) |
Class III devices required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
The labels and packages of Class II medical devices must bear a UDI. § 801.20. |
|
|
Data for class II devices that are required to be labeled with a UDI must be submitted to the GUDID database. §830.300. |
|
|
5 years after publication of the final rule (September 24, 2018) |
A Class II device that is required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
The labels and packages of Class I medical devices and devices that have not been classified into Class I, Class II, or Class III must bear a UDI. § 801.20. Dates on the labels of all devices, including devices that have been excepted from UDI labeling requirements, must be formatted as required by § 801.18. |
|
|
Data for Class I devices and devices that have not been classified into Class I, Class II, or Class III that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. Class I stand-alone software must provide its UDI as required by § 801.50(b). |
|
|
7 years after publication of the final rule (September 24, 2020) |
Class I devices, and devices that have not been classified into Class I, Class II, or ClassIII that are required to be labeled with a UDI, must a bear UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
Compliance dates for all other provisions of the final rule. Except for the provisions listed above, FDA requires full compliance with the final rule as of the effective date that applies to the provision. |
|
Accredited Issuing Agencies
An issuing agency is an FDA-accredited organization that operates a system for assignment of UDIs according to the final rule. The final rule permits multiple issuing agencies and provides a process through which an applicant would seek FDA accreditation as an issuing agency.
Applicants seeking initial FDA accreditation as an issuing agency shall notify the FDA via email at udi@fda.hhs.gov.
FDA has accredited the agencies listed below:
- Firm Name: GS1
Address: Princeton Pike Corporate Center, 1009 Lenox Drive, Suite 202, Lawrenceville, NJ 08648
Contact Person: Siobhan O’Bara, Senior Vice President - Industry Engagement
Phone: (609) 620-8046
Email: sobara@gs1us.org
Web Site: http://www.gs1.org
Date of Initial Accreditation: December 17, 2013
Initial Accreditation Granted through: December 17, 2016 - Firm Name: Health Industry Business Communications Council (HIBCC)
Address: 2525 E. Arizona Biltmore Circle, Suite 127, Phoenix, AZ 85016
Contact Person: Robert A. Hankin, PhD., President and CEO
Phone: (602) 381-1091
Email: rhankin@hibcc.org
Web Site: http://www.hibcc.org
Date of Initial Accreditation: December 26, 2013
Initial Accreditation Granted through: December 26, 2016- Application (pdf)
- Approval Letter(pdf)
- Firm Name: ICCBBA
Address: PO Box 11309, San Bernardino, CA 92423-1309
Contact Person: Pat Distler, Technical Director
Phone: (909) 793-6516
Email: pat.distler@iccbba.org
Web Site: http://www.iccbba.org
Date of Initial Accreditation: February 12, 2014
Initial Accreditation Granted through: February 12, 2017- Application (pdf)
- Approval Letter(pdf)
Accordion UDI
UDI
Unique Device Identification - UDI
A UDI is a unique numeric or alphanumeric code that consists of two parts:
- a device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device, and
- a production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- the lot or batch number within which a device was manufactured;
- the serial number of a specific device;
- the expiration date of a specific device;
- the date a specific device was manufactured;
- the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
As part of the UDI system, the FDA is also creating the Global Unique Device Identification Database (GUDID) which will include a standard set of basic identifying elements for each device with a UDI. Most of this information will be made available to the public so that users of a medical device can easily look up information about the device. The UDI does not indicate, and the database will not contain, any information about who uses a device, including personal privacy information.
The FDA has issued Global Unique Device Identification Database (GUDID) - Guidance for Industry and FDA Staff (pdf)to give labelers an overview of the GUDID. This guidance is designed to help labelers prepare to submit information to the GUDID by describing key GUDID concepts such as accounts, user roles, the device identifier record life cycle, package configurations, and the GUDID data attributes and descriptions.
BENIFITS OF UDI
Benefits of Unique Device Identification
When fully implemented, the UDI system can:
- Allow more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
- Reduce medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
- Enhance analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries. A more robust post market surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
- Provide a standardized identifier that will allow manufacturers, distributors and healthcare facilities to more effectively manage medical device recalls.
- Provide a foundation for a global, secure distribution chain, helping to address counterfeiting and diversion and prepare for medical emergencies.
- Lead to the development of a medical device identification system that is recognized around the world.
IPM & UDI
IPM & UDI
Manage UDI standards with minimal impact to your overall manufacturing and quality processes.
Sharply enforced and ever-evolving UDI regulations shed light on existing gaps, workarounds and inefficiencies within labeling processes, across an enterprise.
As a result of uncovering such setbacks, UDI regulations should serve as a catalyst, and business justification, to improve or simplify operational processes.
The enLabel IPM Platform will continuously support UDI standards by allowing you to:
- Dynamically include production specific and product specific details in the identifier (lot + item).
- Use flexible bar coding standards (GS1, EAN, RFID, 2D and 1D) with minimal impact to operations.
- Deploy trace & track capability throughout the entire supply chain, from received goods to distribution to the point of use.
- Prepare for 2016 and beyond with minimal changes to your current manufacturing process.
- Achieve zero defects in your labeling, documentation and packaging processes.
COMPLIANCE DATES
Compliance Dates for UDI Requirements
The table below outlines key compliance dates in the UDI final rule.
| Compliance Date | Requirement |
|---|---|
|
1 year after publication of the final rule (September 24, 2014) |
The labels and packages of Class III medical devices, and devices licensed under the Public Health Service Act (PHS Act) must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. Data for these devices must be submitted to the GUDID database. § 830.300. A 1-year extension of this compliance date may be requested under § 801.55; such a request must be submitted no later than June 23, 2014. Class III stand-alone software must provide its UDI as required by § 801.50(b). |
|
2 years after publication of the final rule (September 24, 2015) |
The labels and packages of implantable, life-supporting, and life-sustaining devices must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. |
|
A device that is a life-supporting, or a life-sustaining device that is required to be labeled with a UDI must a bear UDI as a permanent marking on the device itself if the device is intended to be used more than once and intended to be reprocessed before each use. § 801.45. Stand-alone software that is a life-supporting or life-sustaining device must provide its UDI as required by § 801.50(b). |
|
|
Data for implantable, life-supporting, and life-sustaining devices that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. |
|
|
3 years after publication of the final rule (September 24, 2016) |
Class III devices required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
The labels and packages of Class II medical devices must bear a UDI. § 801.20. |
|
|
Data for class II devices that are required to be labeled with a UDI must be submitted to the GUDID database. §830.300. |
|
|
5 years after publication of the final rule (September 24, 2018) |
A Class II device that is required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
The labels and packages of Class I medical devices and devices that have not been classified into Class I, Class II, or Class III must bear a UDI. § 801.20. Dates on the labels of all devices, including devices that have been excepted from UDI labeling requirements, must be formatted as required by § 801.18. |
|
|
Data for Class I devices and devices that have not been classified into Class I, Class II, or Class III that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. Class I stand-alone software must provide its UDI as required by § 801.50(b). |
|
|
7 years after publication of the final rule (September 24, 2020) |
Class I devices, and devices that have not been classified into Class I, Class II, or ClassIII that are required to be labeled with a UDI, must a bear UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
|
Compliance dates for all other provisions of the final rule. Except for the provisions listed above, FDA requires full compliance with the final rule as of the effective date that applies to the provision. |
|
ISSUING AGENCIES
Accredited Issuing Agencies
An issuing agency is an FDA-accredited organization that operates a system for assignment of UDIs according to the final rule. The final rule permits multiple issuing agencies and provides a process through which an applicant would seek FDA accreditation as an issuing agency.
Applicants seeking initial FDA accreditation as an issuing agency shall notify the FDA via email at udi@fda.hhs.gov.
FDA has accredited the agencies listed below:
- Firm Name: GS1
Address: Princeton Pike Corporate Center, 1009 Lenox Drive, Suite 202, Lawrenceville, NJ 08648
Contact Person: Siobhan O’Bara, Senior Vice President - Industry Engagement
Phone: (609) 620-8046
Email: sobara@gs1us.org
Web Site: http://www.gs1.org
Date of Initial Accreditation: December 17, 2013
Initial Accreditation Granted through: December 17, 2016 - Firm Name: Health Industry Business Communications Council (HIBCC)
Address: 2525 E. Arizona Biltmore Circle, Suite 127, Phoenix, AZ 85016
Contact Person: Robert A. Hankin, PhD., President and CEO
Phone: (602) 381-1091
Email: rhankin@hibcc.org
Web Site: http://www.hibcc.org
Date of Initial Accreditation: December 26, 2013
Initial Accreditation Granted through: December 26, 2016- Application (pdf)
- Approval Letter(pdf)
- Firm Name: ICCBBA
Address: PO Box 11309, San Bernardino, CA 92423-1309
Contact Person: Pat Distler, Technical Director
Phone: (909) 793-6516
Email: pat.distler@iccbba.org
Web Site: http://www.iccbba.org
Date of Initial Accreditation: February 12, 2014
Initial Accreditation Granted through: February 12, 2017- Application (pdf)
- Approval Letter(pdf)
